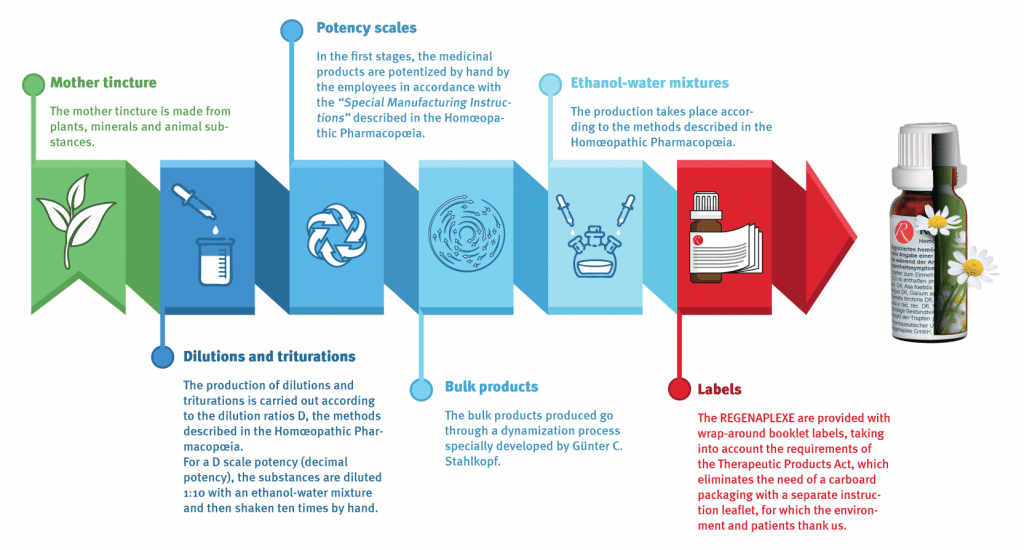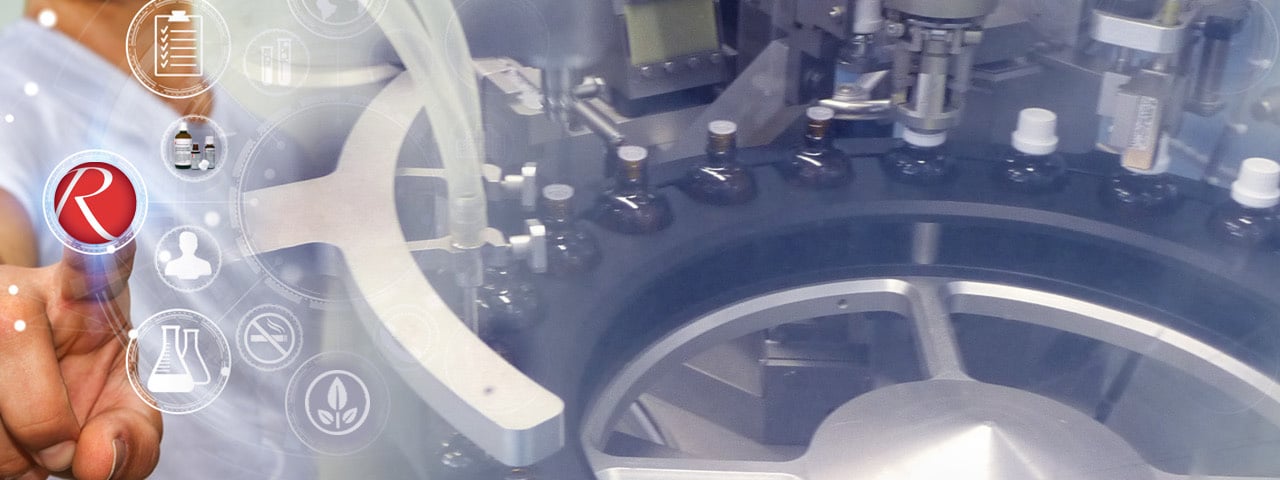
Production of REGENAPLEX® remedies
Production is carried out using the formulations developed by Günter C. Stahlkopf
Mother tinctures, tinctures and triturations are obtained from carefully sourced and certified suppliers. Homeopathic pharmaceuticals are manufactured in Switzerland according to the German Homeopathic Pharmacopoeia (HAB) using the following raw materials:
- Fresh plants or parts of fresh plants
- Dried plants or parts of dried plants
- Animal substances
- Mineral and inorganic raw material
ainsi que les agents et excipients prévus dans le HAB tels que : éthanol absolu, eau, glycerol, monohydrat de lactose.
Production Steps
Dilutions and triturations
Production is carried out using dilution factor D as specified in the German Homeopathic Pharmacopoeia (HAB). For a D-Potentiation substances are diluted by a mixture of Ethanol-Water with the factor 1:10 and thereafter are shaken by hand 10 times.
Potentiation steps
The first steps of Potentiation are carried out manually as specified in the German Homeopathic Pharmacopoeia (HAB).
Bulk Production Batches
Produced bulk batches are treated by a special dynamisation-procedure developed by Günter C. Stahlkopf.
Purified water
Water is purified in three steps (demineralization, reverse osmosis, electro-deionisation); the purified water meets the specifications of the European Pharmacopoeia (Ph. Eur.).
Ethanol-alcohol water mixture
Production follows the methods specified in the Homeopathic Pharmacopoeia (HAB).
Labelling
Labelling conforms to specifications of the Swiss “Heilmittelgesetz (law on medical products)” . Labels with necessary information/instructions are attached directly to the bottles. For ecological reasons we do without outer packages and instruction leaflets.
Finished product
Bottles are filled, the medicine dropper inserted and bottles sealed on a filling line under sterilisation by a laminar-flow unit, followed by a 100 % control of the filling weight.
Quality Control
All raw materials received are thoroughly examined at the reception point and the end product is released upon approval. Testing is carried out according to the monographs of the German Homeopathic Pharmacopoeia (HAB), the European Pharmacopoeia (Ph. Eur.) and REGENA’s own specifications.
Stability testing and on-going stability testing of the starting material and of the finished products, in order to determine their durability, is carried out according to stability testing requirements, prescribed in REGENA AG durability specifications.
Quality Assurance
Product Quality Review (PQR)
To ensure product quality, all data concerning quality and production are assembled and analysed by means of a product quality review.
Maintenance
Equipment and facilities relevant for GMP are regularly calibrated, checked and serviced to ensure flawless functionality.